Area of focus #1
Drugs & medical devices
Additional areas of focus
Food
Education
Research and epidemiology
Prevention
Goal
Ensure that people with alpha-gal syndrome (AGS) have access to safe drugs and medical devices
“Numerous foods and pharmaceuticals with components of mammalian origin contain α-Gal and may trigger reactions, which is why this allergic disease is known as the α-Gal syndrome, AGS.” (144)
Priority #1
Label drugs and select medical devices
that contain alpha-gal
Challenge
The scope of the challenge
- There is a critical need for legislation requiring disclosure of alpha-gal in drugs and medical devices to protect the welfare of people with alpha-gal syndrome.
- The CDC estimates that alpha-gal syndrome (AGS) affects up to 450,000 people in the U.S., making it the 10th most common food allergy.8,9However, an evidence-based approach to decision-making about the disclosure of food allergens in medical products calls for more than estimating the number of people affected by different food allergies. It also requires consideration of data on the magnitude of the threat posed by different food allergens in drugs and medical products.
- Unlike almost all other food allergens, including the top nine food allergens,75 alpha-gal is also a major drug excipient allergen and a top cause of perioperative and drug-related anaphylaxis.74,76,77,78,81-118,136 For this reason, allergy to alpha-gal isn’t a simple food allergy and is considered a syndrome.73,132
- Hundreds of thousands of drugs and medical devices are mammal-derived or contain mammal-derived active or inactive ingredients. No complete list of these products exists. Some examples include tens of thousands of over-the-counter and prescription drugs; 120 vaccines; biologics, such as cetuximab and infliximab; anti-venom; suppositories and vaginal capsules; topical products; an unknown number of products used in perioperative settings, such as heparin; bioprosthetic heart valves; extracellular matrix; heart patches; hernia mesh; hemostatic agents; plasma volume expanders; sutures; lubricants; growth factors; viscosurgical devices; thrombin glues; and products like propofol and intralipid when the glycerin they contain is mammal-derived. .13,60,79,81-118,136,137
- According to a recent DailyMed search, over 64% of the drugs in the DailyMed database (over 230,000 products) contain active or inactive ingredients that are either mammal-derived or potentially mammal-derived. 120,125
- Some alpha-gal-containing drugs are even more dangerous for allergic patients than mammalian meat. 4,5,6,119
Lack of access to critical information
- Patients and providers are usually unaware of the mammalian origin of ingredients and products. For example, most providers do not know that magnesium stearate, chondroitin, hyaluronidase, lactoferrin, glycerin, casamino acid, thrombin, pancrelipase, or even collagen, gelatin, and heparin are derived from mammals.
- Moreover, in the case of biologics made using mammalian cell lines, disclosure of ingredients alone is insufficient for determining alpha-gal content. This is because alpha-gal in these products is an integral component of the molecules that make up the active ingredient.60,121-124,138
- The only way for patients and providers to know if biologics contain alpha-gal is if they are tested for it and this information is disclosed.60,121-124,138 Currently, this is not the case.
- At present, approximately 80% of the currently approved recombinant therapeutic proteins are produced by Chinese hamster ovary cells, which variably glycosylate with alpha-gal.138,139
- This makes the use of new biologics Russian roulette for people with AGS.13
The dangers
- More than 75% of people with AGS have had allergic reactions to medications.10
- Half of all people with AGS have had anaphylactic reactions to drugs or other health products.10
- Numerous life-threatening AGS-related reactions to drugs and medical products and other adverse outcomes, such as premature degeneration of bioprosthetic valves and implants, have been documented.81-118
- More than 10 deaths associated with alpha-gal reactions to drugs have been documented, and there are anecdotal reports of additional deaths.4,5,6
Opportunities
- Amend the Federal Food, Drug, and Cosmetic Act to require the label of a drug intended for human use to identify each ingredient in such drug that is derived, directly or indirectly, from a mammal (excluding non-catarrhine primates and mammals genetically modified not to express alpha-gal), red algae (Rhodophyta), or other known sources of alpha-gal.
- Require the label of select medical devices intended for human use that have the potential to expose patients to alpha-gal to identify each ingredient in such drug that is derived, directly or indirectly, from a mammal (excluding non-catarrhine primates and mammals genetically modified not to express alpha-gal), red algae (Rhodophyta), or other known sources of alpha-gal. Examples of such devices include heparin flushes, extracellular matrix, bioprosthetic heart valves, bandages with mammal-derived adhesive, and collagen sutures.
- Require that biologics, vaccines, and other medical drugs and devices made in non-catarrhine primate*, mammal-derived cell lines, including chimeric human/mammal cell lines, be tested for alpha-gal above a standardized, detectable level. Additionally, require that this information be disclosed publicly, including on the labels of these products.
- Allow labeling of drugs and dietary supplements to include an accurate and truthful certification symbol denoting that the product(s) are free of animal-derived ingredients.
- In the absence of the above legislation, as a stop-gap measure:
- Mandate that the FDA create and maintain a publicly funded and accessible database with information about the alpha-gal content of drugs and other medical products or fund a private entity to do this.
- Mandate that the FDA create a dedicated, drug information service to assist providers and patients with information about drug safety related to alpha-gal syndrome or fund a private entity to do this.
*Non-catarrhine primates include all primates except human, great apes, and Old World monkeys.
Alignment with National public health strategies and expert recommendation
- Dozens, if not hundreds, of papers have detailed the everyday and perioperative risks of mammal-derived medical products for people with alpha-gal syndrome. Two of the more prominent of these include:
- Dunkman WJ, Rycek W, Manning MW. What does a red meat allergy have to do with anesthesia? Perioperative management of alpha-gal syndrome. Anesthesia & Analgesia. 2019 Nov 1;129(5):1242-8.
- Nourian MM, Stone CA Jr, Siegrist KK, Riess ML. Perioperative implications of patients with alpha-gal allergies. J Clin Anesth. 2023;86:111056.
- See References for many more papers on the risks of mammal-derived medical products for people with alpha-gal syndrome.
- In his 2020 paper “Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients,” expert Scott Commins, MD, PhD identified the lack of adequate labeling for mammalian-derived sources in foods, medications, and vaccines as a challenge to the management of AGS.13
- In its 2020 and 2022 reports to Congress, the Congressionally mandated Tick-Borne Disease Working Group called for the labeling of pharmaceuticals that contain non-primate mammalian ingredients (active or inactive).129
- In their 2024 paper “Hypersensitivity reactions due to North American pit viper antivenom administration and confirmed elevation of alpha-gal IgE,” Banner et al called for clear labeling of alpha-gal-containing pharmaceuticals.142
Due to the ubiquitous inclusion of mammal-derived materials in foods, medications, personal products and stabilizing compounds, full avoidance is difficult to achieve.
As with management for any food allergy, AGS management is based on allergen avoidance. For patients with AGS, however, this tenet of self-protection is made difficult by the lack of adequate labeling for mammalian-derived sources in foods, medications, and vaccines.
Because patients with AGS can display symptoms after exposure to not just meat, but also medications and inactive ingredients derived from animals, there is a potential risk for unintentional intraoperative exposure.
Every medication used in an AGS patient in the entire perioperative period must be screened in order to limit exposure to mammalian products.
Mammalian-derived by-products are common in medications and devices, both as active and inactive ingredients. This poses a potential safety risk during receipt of healthcare services.
Inactive ingredients such as magnesium stearate, glycerin and gelatin may be made from animal, plant or synthetic sources. Sources for inactive ingredients may vary by the manufacturer lot number (identifier assigned to a batch of medications during the manufacturing process) since companies are not obligated to report the source to any agency. If there is no known safe alternative, the manufacturer should be contacted and asked if the source (animal vs synthetic) is known. Unfortunately, many companies do not have this information easily available and may not be able to supply this information for several days.
Priority #2
Determine the risks associated with drugs and medical products that contain or potentially contain alpha-gal

Challenge
- There is a lack of data on the risk of reactions to the wide variety of products that may include small amounts of alpha-gal.13,60,116
- This lack of data creates uncertainties and risks for AGS patients and providers.13,60,116
- Research and the development of tools to better study, characterize, and measure alpha-gal content in drugs and select medical devices that have the potential to expose patients to alpha-gal are needed.
Opportunities
- Secure federal funding for research to characterize and measure the presence of alpha-gal in drugs, select medical devices that have the potential to expose patients to alpha-gal, and mammal-derived ingredients commonly used in their production.
- Secure federal funding to quantify risks associated with the use of products that contain or potentially contain alpha-gal.
- Secure federal funding for the development of tools and protocols for use in the characterization and measurement of the presence of alpha-gal in drugs, select medical devices that have the potential to expose patients to alpha-gal, and mammal-derived ingredients commonly used in their production.
Alignment with National public health strategies and expert recommendation
- In “Diagnosis and management of patients with the α-Gal syndrome,” experts Thomas Platts-Mills, MD, PhD and Jeffrey Wilson, MD, PhD identified a need to better understand the risk of reactions to the wide variety of products that may include small amounts of material derived from mammals.60
- In “Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients,” expert Scott Commins, MD, PhD also identified a need to better understand the risk of reactions to the wide variety of products that may include small amounts of material derived from mammals.60
- In “Perioperative implications of patients with alpha gal allergies,” Nourian et al describe a need to determine the alpha-gal content of mammal-derived excipients such as glycerin, magnesium stearate, and stearic acid.116
We also need to better understand the risk of reactions to the wide variety of products that may include small amounts of material derived from mammals.
Priority #3
Accelerate the development and approval of alpha-gal-friendly drug and medical device alternatives e.g. heparin and bioprosthetic heart valves made from alpha-gal knockout pigs
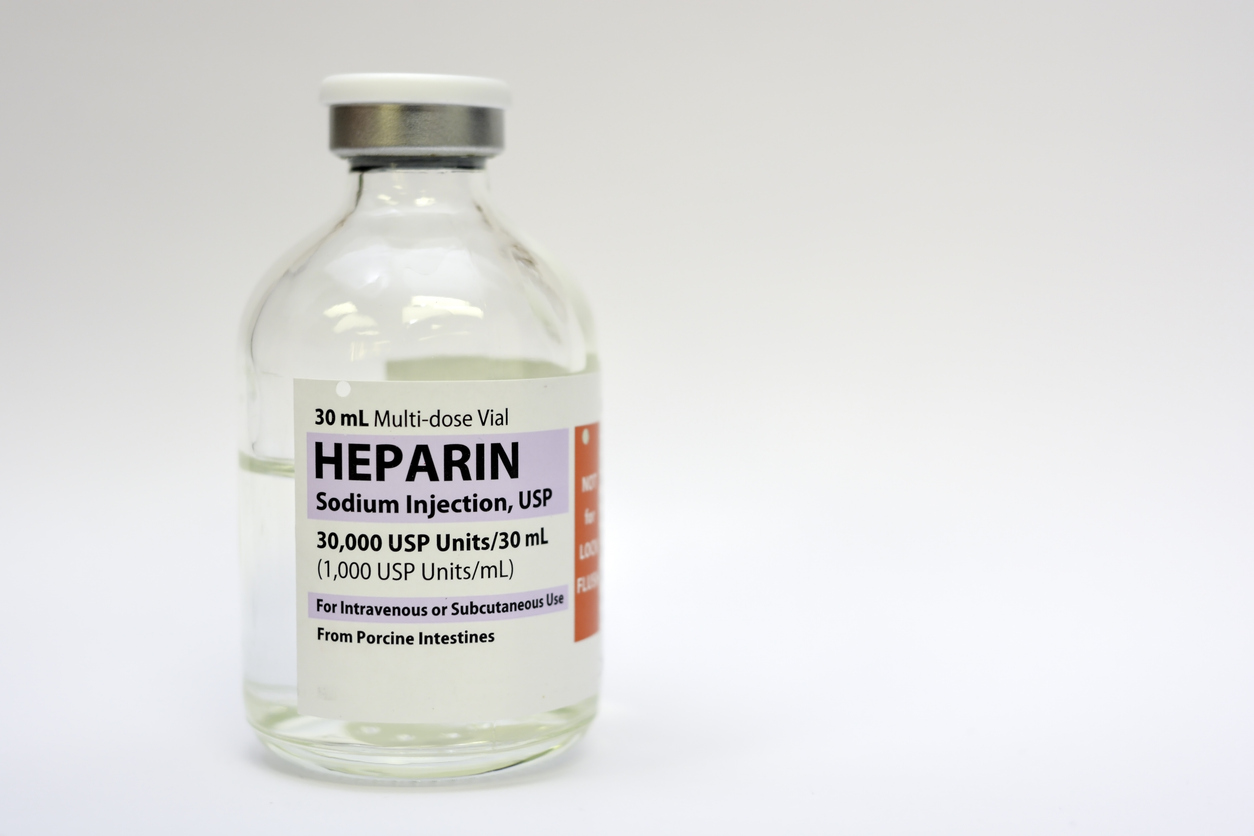
Challenge
- The use of porcine- and bovine-derived drugs and medical devices and drugs and medical devices made with porcine- and bovine-derived ingredients can lead to adverse outcomes in people with alpha-gal syndrome, 81-118 such as:
- Life-threatening anaphylactic reactions81-118
- Hyperacute rejection or premature degeneration of implants81-118
- Some examples of these products include:81-118
- Heparin
- Gelatin/collagen
- Bioprosthetic heart valves
- Heart patches
- Collagen scaffolding
- Extracellular matrix scaffolding
- Hernia mesh
- Orthopedic and other implants
- Hemostatic agents and other gelatin-based products
- Pancreatic enzyme replacements
- GalSafe pigs, produced by Revivicor, contain a genetic modification intended to eliminate alpha-gal sugar on the surface of the pigs’ cells.
- The use of GalSafe pigs and other alpha-gal knock-out mammals for production of porcine-derived drugs and medical devices would improve their safety for people with AGS.
- In 2020, the FDA approved GalSafe pigs both for potential therapeutic uses and for food.127
- However, the cost of bringing individual, GalSafe medical products to market is currently prohibitive.128
- Alternatives to the use of mammalian cell lines that glycosylate with alpha-gal, such as CHO, Sp2/0, and NS0, for the production of biologics also need to be explored.6, 79,84,107-110 ,121-4
Opportunities
- Secure federal funding to support the development of drugs and medical devices made from GalSafe or other alpha-gal knockout mammals, such as heparin, collagen, gelatin, dermis, heart valves and other implants.
- Expedite the approval process of GalSafe medical products.
Alignment with National public health strategies and expert recommendation
- The 2020 Alpha-Gal Syndrome Subcommittee Report to the Tick-Borne Disease Working Group
alpha-gal sub identified the use of alpha-gal deficient pigs as a source of heart valves as a potential solution to problems related to the use of bioprosthetic heart valves in people with AGS. - In “Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients,” expert Scott Commins, MD, PhD identified approval of alpha-gal free porcine products as an effective way to develop ‘AGS-safe’ foods, medications, and implantable devices.13
- In their 2024 paper “Hypersensitivity reactions due to North American pit viper antivenom administration and confirmed elevation of alpha-gal IgE,” Banner et al called for improved antivenom products to reduce alpha-gal-related reactions.142
Approval of alpha-gal-free porcine products would represent an effective way to develop ‘AGS-safe’ foods, medications, and implantable devices.
